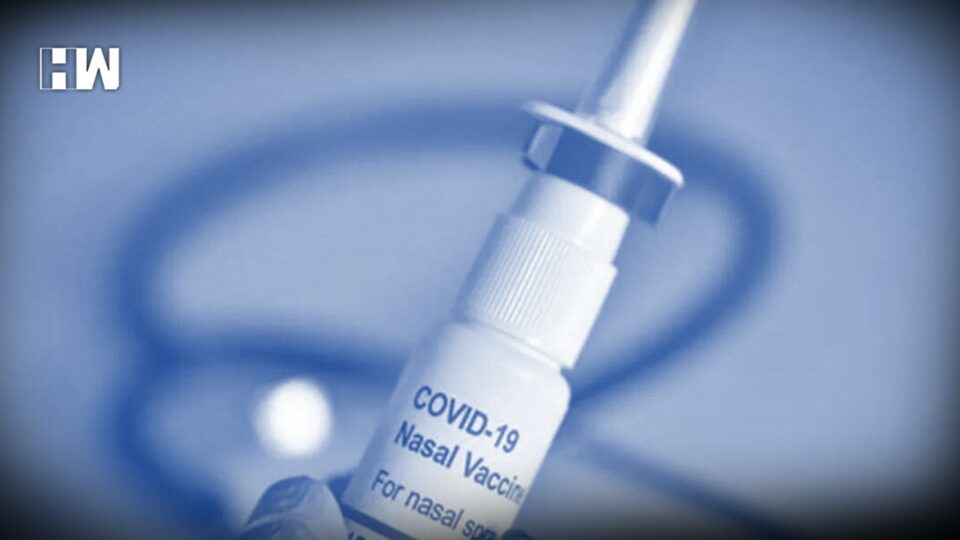
The vaccine was designated as the “World’s First Intra-Nasal COVID Vaccine” developed by India by the Ministry of Science
New Delhi: With the new variant of COVID there is a surge in cases worldwide. With the new Omicron sub-variant, India too is updating its COVID laws. Union Health Ministry too gave a green signal for Bharat Biotech’s intranasal Covid vaccine.
The intranasal vaccine will now be included in the vaccination programme as a booster dose for those above 18 years of age. The needle-free vaccine will be available at private centers. On Friday evening it will be introduced on the Co-WIN platform as well.
In November, the Drugs Controller General of India gave approval for the restricted use of vaccine – BBV154 – under emergency situation for adults only.
According to Bharat Biotech, there are many advantages of this vaccine. They say that the nasal route has excellent potential for vaccination due to the organized immune systems of the nasal mucosa. The vaccine is non-invasive, needle-free, and can be administered quickly and easily by anyone as it does not requires trained health professionals. The monetary amount to be paid for the administration of the vaccine is yet to be decided.
Health Minister Dr. Mansukh Mandaviya said that Bharat Biotech nasal vaccine has been approved as a booster dose.
Prime Minister Narendra Modi on Thursday afternoon held a high-level meeting to review the Covid-19 situation and its related aspects in the county.
In the last six months, India reported four cases of the BF.7 Omicron sub-variant, which is driving the current surge of infections in China. Sources said there are currently 10 different variants of Covid-19 in the country, with the latest being BF.7.
Mask use and social distancing will be necessary during flights and at the airport entry and exit points. Those who exhibit symptoms will be separated from other travelers and transferred to an isolation facility for further care.
Union Health Minister Mansukh Mandaviya on Thursday said that the government has started random RT-PCR sampling among the passengers arriving at International airports in the country amid the recent surge of Coronavirus infection in various countries including China, Japan, South Korea, France, and the United States.
“We have also started the random RT-PCR sampling among passengers arriving at International airports in the country. We are committed to tackling the pandemic and are taking appropriate steps,” Health Minister Mansukh Mandaviya said in his statement in the Lok Sabha while advising the States to make sure that people wear masks, use sanitizers and maintain social distancing even during the festive and the New Year season. He also encouraged the states to increase awareness towards precautionary doses against Coronavirus.
As of now, Bharat Biotech’s Covaxin, Serum Institute’s Covishield, Covovax, Russian Sputnik V, and Biological E Ltd’s Corbevax are listed on the Co-WIN portal. The vaccine was designated as the “World’s First Intra-Nasal COVID Vaccine” developed by India by the Ministry of Science. In partnership with Washington University St Louis, the vaccine was developed.
It was created, developed, and its effectiveness was assessed in preclinical trials by University St. Louis. Bharat Biotech developed products in the areas of preclinical safety assessment, large-scale manufacturing scale-up, formulation, and delivery device development, including human clinical trials.
The testing and trials of this vaccine were partially funded by the Government of India through the Department of Biotechnology’s, Covid Suraksha program.
Also Read: “Modi Father Of New India”: Amruta Fadnavis
Precautionary dose information from the Cowin website reveals a sharp increase in registration and immunization from December 18, in line with the recent outbreak in China and official requests to get immunized. On December 18, about 4,000 persons took the precautionary dose; on December 22, that number rose to 57,000.
Many states have implemented different COVID laws. Karnataka has made it mandatory to wear face masks indoors and in closed spaces like pubs, bars, restaurants, malls, offices, buses, and trains. Anyone who exhibits signs of respiratory problems has been advised to separate themselves and get checked right away. Officials have been told to hurry up with the booster dosage shot.
Kerala Health Minister Veena George said all districts have been told to strengthen surveillance. Testing will be done rapidly on a large scale. In the state genomic sequencing of positive cases would be held. “There won’t be any curb on Christmas-New Year celebrations,” she said.
Chief Minister Eknath Shinde reviewed the COVID situation in the state. He ordered all the ministers to ensure that health facilities are ready in their respective districts. He asked the Chief Secretary to ensure that the five-point programme of — test, track, treat, vaccinate and ensure COVID-appropriate behaviour— is followed in the state.
As an independent media platform, we do not take advertisements from governments and corporate houses. It is you, our readers, who have supported us on our journey to do honest and unbiased journalism. Please contribute, so that we can continue to do the same in future.